Electronics
01
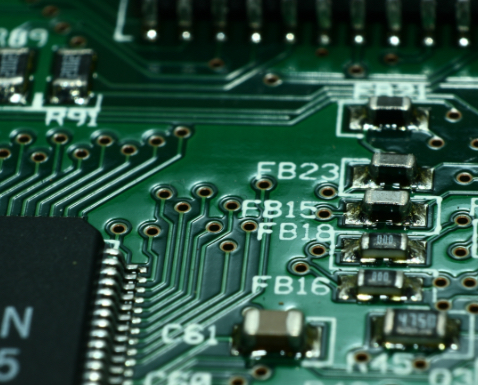
Microchips
Ultrapure or electronic-grade solvents (alcohols, esters and ketones) with deficient levels of metal ions in the solvents are used to produce microchips. Metal ions can cause short circuits that result in poor-quality microchips. Electronic-grade solvents are used to dissolve a photo-sensitive polymer that is then spun on a silicon wafer to produce the micro-circuit.

02
Battery recycling
Solvents blends with hydrocarbon solvents or glycol ethers extract valuable elements or metals like cobalt from the metal oxides commonly used as cathodes in lithium-ion batteries. In many lithium-ion battery recycling process flowsheets, spent batteries are dismantled, and the parts containing the electrodes, such as battery cells, get crushed or shredded to produce a powdery fraction referred to as “black mass.” This black mass comprises electrode coatings (metal oxides and carbon) and, therefore, contains value elements such as graphite, manganese, cobalt, nickel and lithium. Black mass requires further processing to isolate manganese, cobalt, nickel and lithium salts. This is conventionally done through hydrometallurgy, where metals from the black mass are dissolved and then chemically separated by solvent extraction.

03
Cleaning – Circuit & semiconductor boards
Etching is a subtractive method used to produce printed circuit boards: acid is used to remove unwanted copper from a prefabricated laminate. This is done by applying a temporary mask that protects parts of the laminate from the acid and leaves the desired copper layer untouched.
Solvents like acetone are typically used to remove unwanted material from metal and alloy surfaces. Acetone cleans and prepares metallic surfaces before sandblasting, coating or corrosion protection.